Veoneer
New Process Validation
Veoneer Canada Safety Systems, a leading automotive safety supplier, was seeking to commission new production lines to support new model updates and business forecasts. With fixed start-of-production deadlines, the company faced challenges with scaling capacity. Yet, verification and validation were required to ensure the output met zero-defect safety standards.
During my 16 month internship in the NPPI department, I collaborated with a team of 6 multidisciplinary senior engineers to launch 4 new production lines for restraint control safety systems (i.e. pressure and acceleration sensors for airbag deployment).
My work consisted of validating 20+ processes and equipment per line using root cause analysis, sensitivity analysis, Cpk/Gage R&R studies, strain testing, and run-at-rate capacity verification.
Project Overview
New Model Launch & Capacity Expansion
Stakeholders
GM, Ford, Stellantis, Nissan, Mazda, Hyundai, Kia
Technical Environment
Lean Six Sigma, IATF 16949 Compliance (ISO 9001), Automated robotics
Scope of Work
Process validation, Risk Analysis (PFMEA), Statistical Process Control, Strain Analysis
Key Deliverables
Cpk/GRR Studies, Strain Reports, Run-at-rate capacity verification
Process and Work
Process Capability (Cpk) and Gage R&R Studies
To ensure accuracy and repeatability over continuous cycles, Statistical Process Control (SPC) was used to validate each process:
- A minimum of 30, continuous samples were produced, measured, and recorded.
- Minitab was used to perform complex statistical calculations, verify compliance with standard acceptance criteria, and visualize the data to make evidence-based decisions.
- Results were presented for approval to advance project to the next phase.
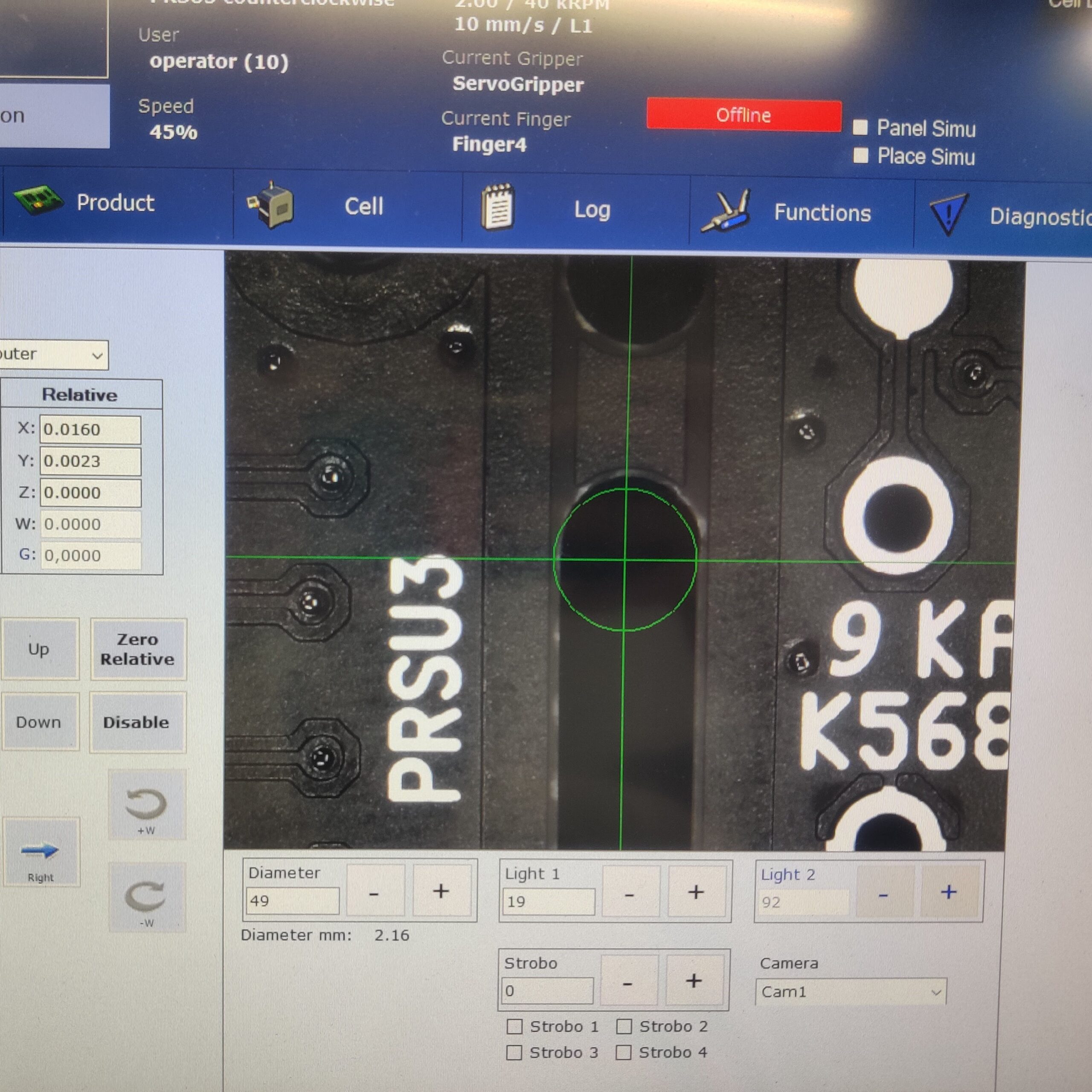


Sensitivity Analysis
Often times, it required extensive troubleshooting and iteration to achieve the desired Cpk and GRR results. Root cause analysis and sensitivity analysis were used to streamline this process.
- The goal was to balance the trade-off between cycle time and quality.
- Variables were controlled and isolated to find the sweet spot.
- Developed intuition and technical understanding of how individual parameters affect the overall system.

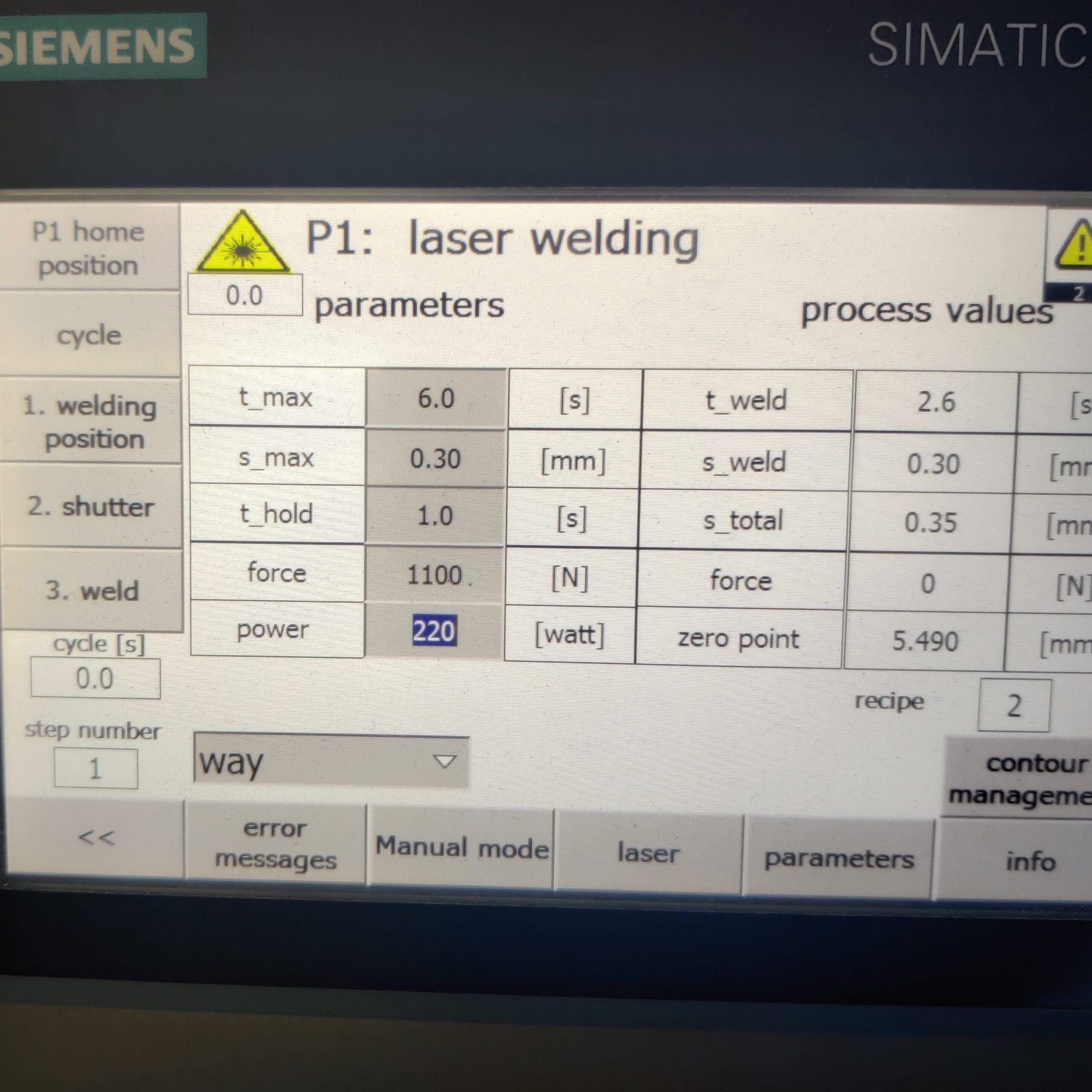
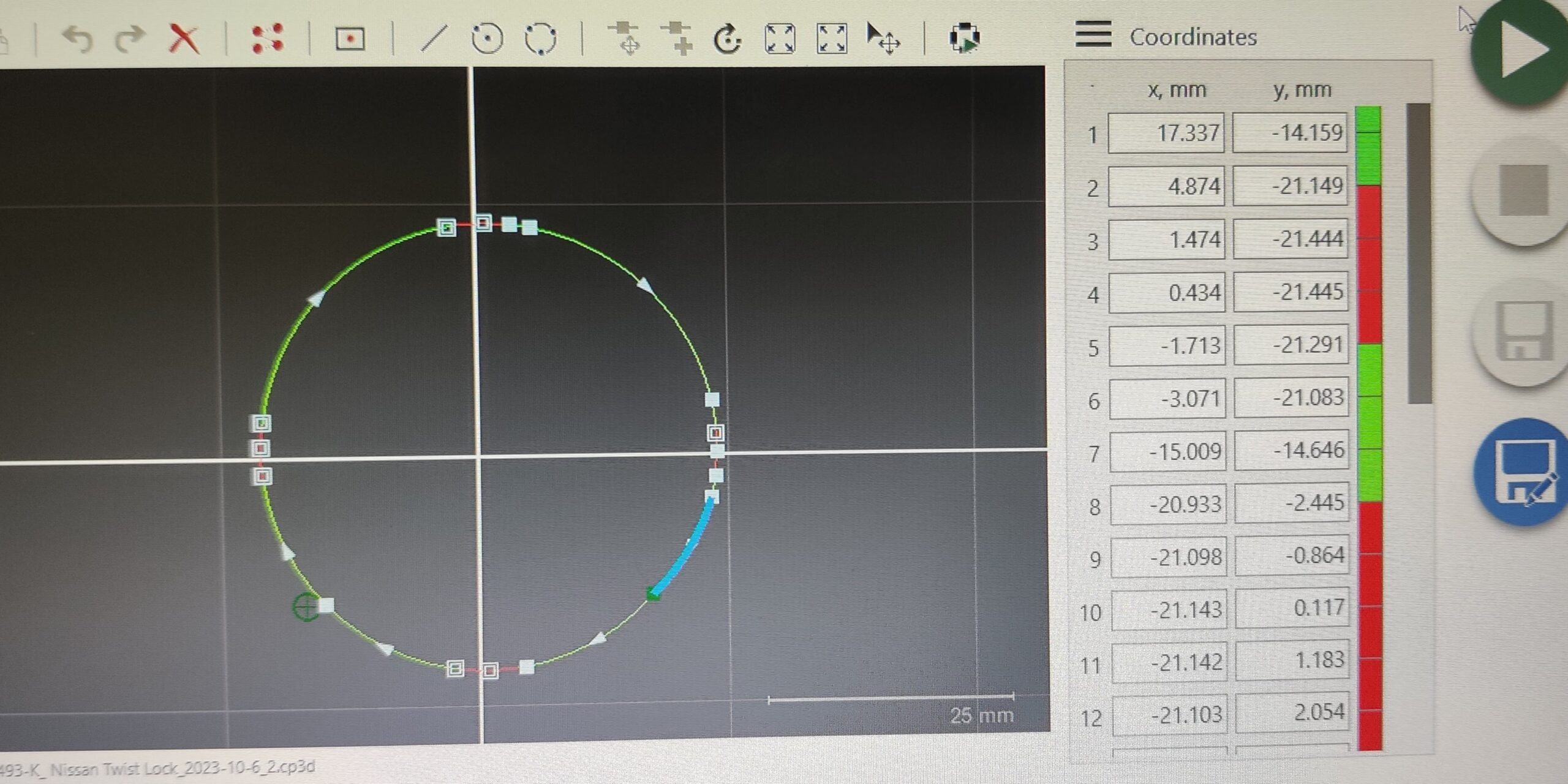
Strain Testing
It’s important airbags deploy at the right time in the event of an accident. The sensor components are sensitive and frequently handled by robotic systems.
- I generated 10 strain test reports for 5 processes that involved physical contact with machinery or tooling.
- Strategically applied strain gages in critical locations while overcoming spatial constraints from moving equipment (pinched wires, tight spaces).
- Learned to interface with PLC and HMI for manual mode access.



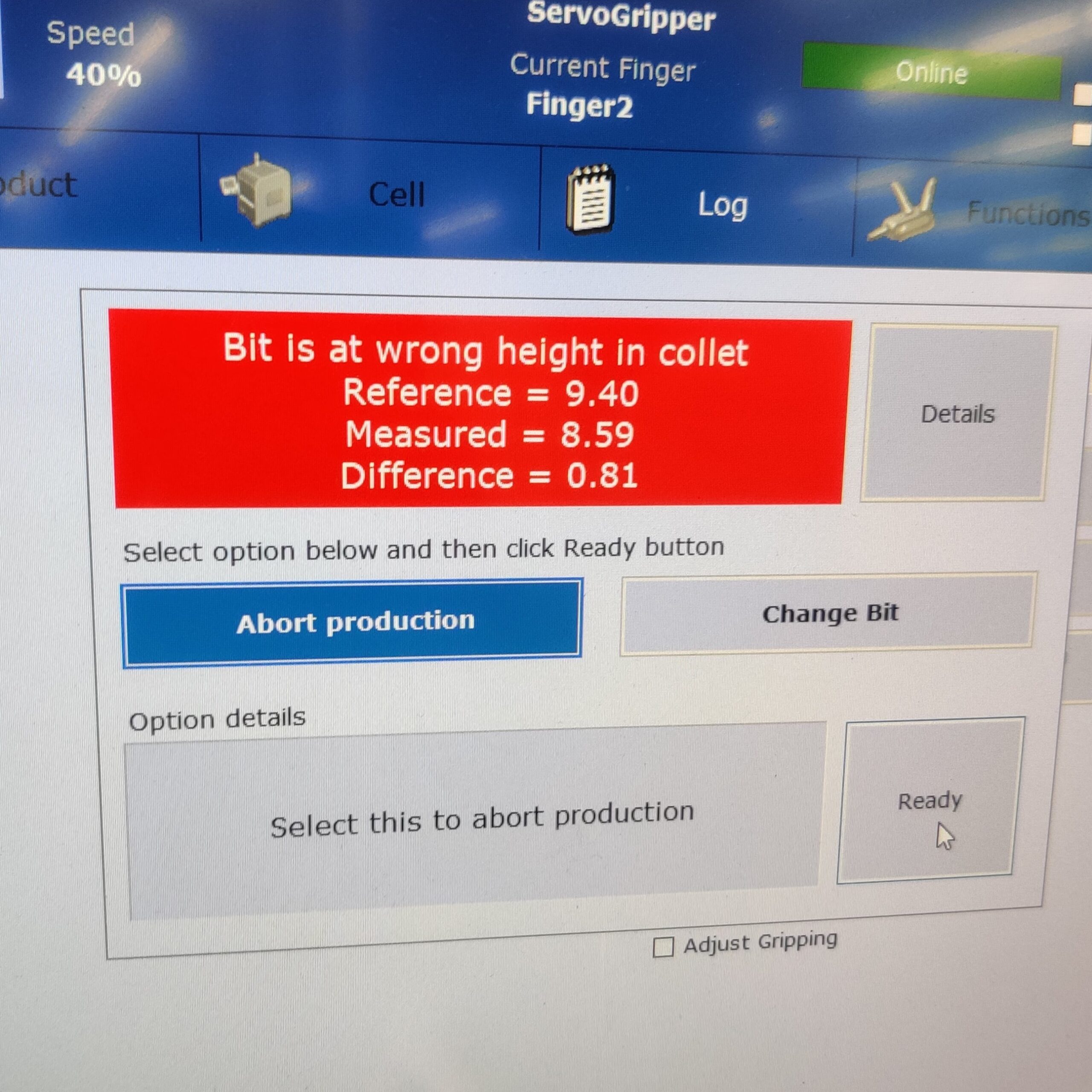
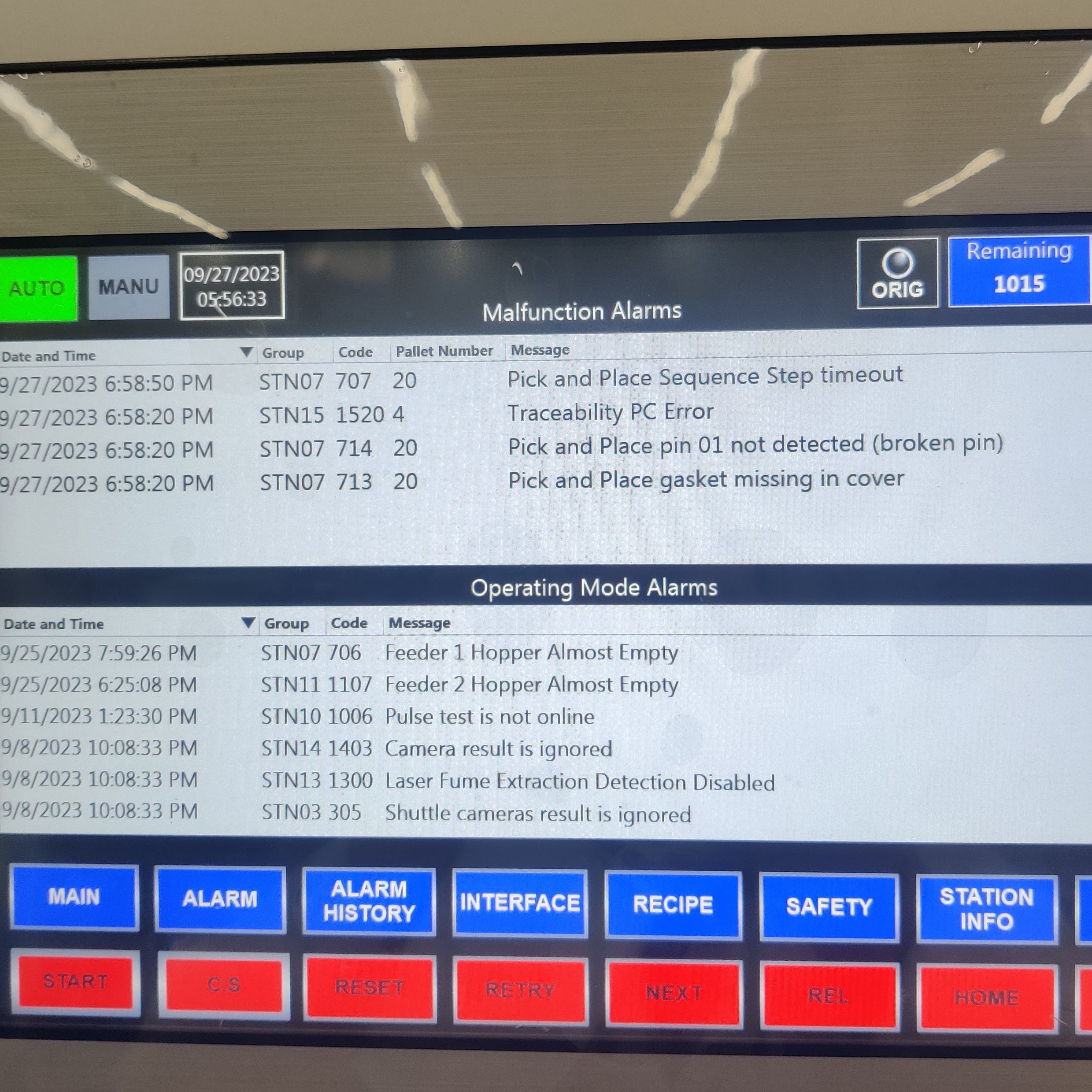
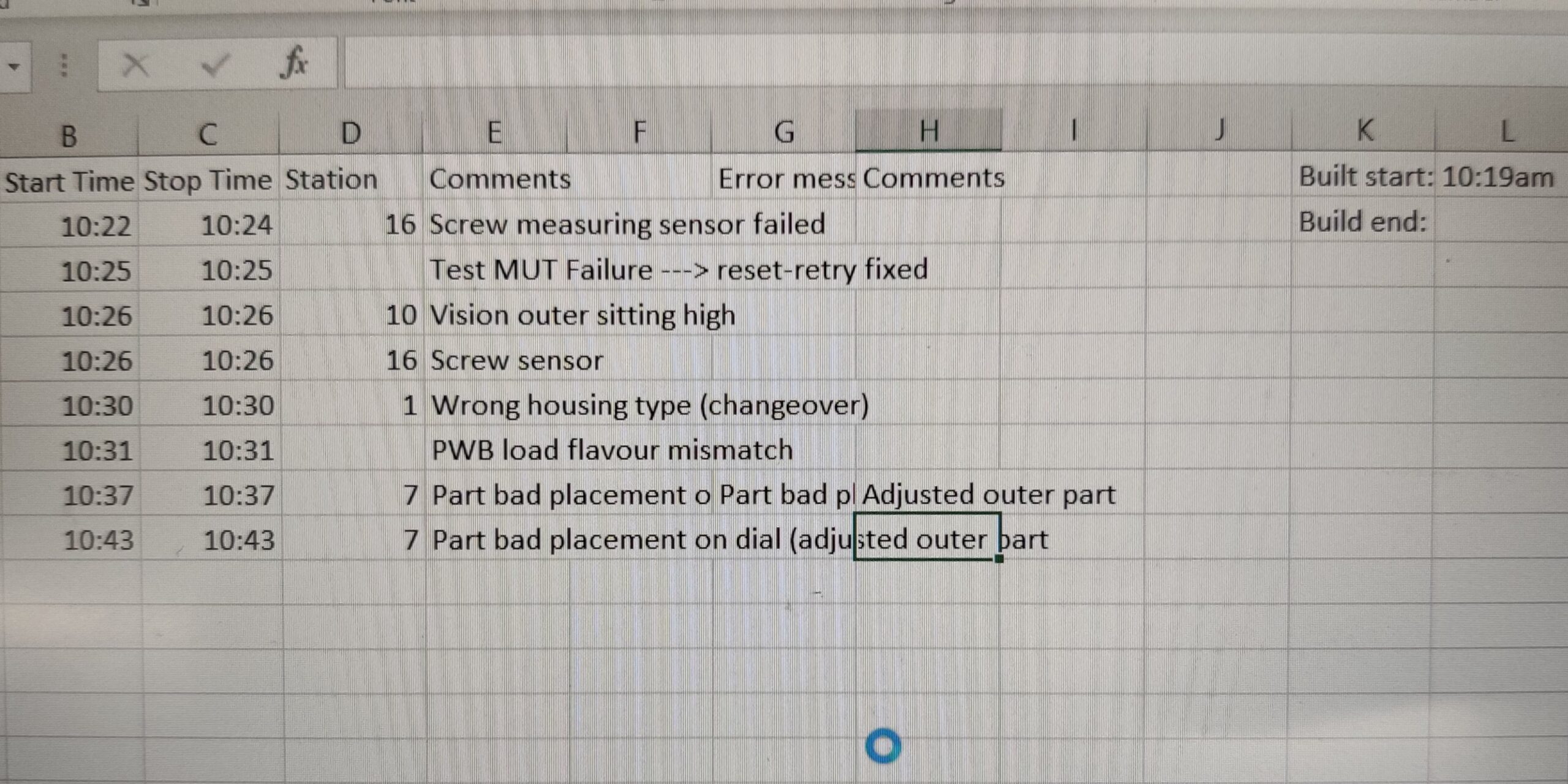
Run-At-Rate Capacity Verification
Once all individual processes were validated, run-at-rate builds were conducted to verify the line could meet the necessary production targets.
- Collaborated with product lead, project lead, test, and controls engineers to ensure the line ran without significant stoppages and met daily required production rate, per client’s forecasts.
- Performed PFMEA audits to ensure verify system’s ability to detect and mitigate process failures; i.e. failure-testing.
Learning Opportunities
Open-ended problem solving
A lot of the work involved troubleshooting with many different ways of approaching it. Often times, the exact cause of an issue with a process or equipment was unknown and required broad approaches to narrow down the problem. I had to pick a starting place based on the current information I had and build from there while being mindful of time and resources.
Working with International clients
Meetings with overseas vendors occasionally required unordinary working hours. For instance, I participated in a virtual Factory Acceptance Test (FAT) in Sweden from 5am to 1pm EST.
Compliance to QMS
All verification & validation for processes and equipment were conducted to comply with internal QMS (IATF:2016, ISO 9001).
Working with complex systems
Each line had around 20 processes and each process had its own set of variable parameters. I had to learn, through trial and error, how isolating and changing 1 variable affected the overall system. Based on these conclusions, I was then able to optimize the system parameters to meet the desired requirements.
Process Capability (Cpk) and Gage R&R Studies
To ensure accuracy and repeatability over continuous cycles, Statistical Process Control (SPC) was used to validate each process:
- A minimum of 30, continuous samples were produced, measured, and recorded.
- Minitab was used to perform complex statistical calculations, verify compliance with standard acceptance criteria, and visualize the data to make evidence-based decisions.
- Results were presented for approval to advance project to the next phase.





Process Capability (Cpk) and Gage R&R Studies
To ensure accuracy and repeatability over continuous cycles, Statistical Process Control (SPC) was used to validate each process:
- A minimum of 30, continuous samples were produced, measured, and recorded.
- Minitab was used to perform complex statistical calculations, verify compliance with standard acceptance criteria, and visualize the data to make evidence-based decisions.
- Results were presented for approval to advance project to the next phase.

